27th May 2022
The BEAI Executive Committee calls for applications / nominations for the 2022 BEAI Early Career Award. The prize will be awarded for the first time at the BEAI Annual Scientific Conference in the Aviva Stadium, Dublin on 30th September 2022. The Early Career Award is given in recognition of a single achievement in Biomedical / Clinical Engineering. The total prize value amounts to €2000 and is reserved for members of the Biomedical / Clinical Engineering Association of Ireland whose age is less than 35 on July 31st of the year of the Conference. The applicaiton / nomination should include the name of the candidate accompanied by a brief characterization of the work singled out for the prize. Members of the BEAI should submit their application / nomination via the BEAI website www.beai.ie

To become a member of the BEAI please visit https://www.beai.ie/content/individual-membership
Individual member benefits of the BEAI include:
- Each member is contributing to the support and development of Biomedical and Clinical Engineering within Ireland and internationally, by enabling the BEAI to participate as a full member of the IFMBE
- BEAI members are entitled to a free copy of the Spectrum Journal
- BEAI members are welcome to present at the BEAI Annual Scientific Conference and receive a discount on the entry fee
- BEAI members have free access to the member’s only section of the BEAI website, giving them access to the archived and latest editions of the Spectrum Journal
- BEAI members have access to the BEAIs Continuing Education Series
- BEAI members have access to the BEAIs online discussion forum
- BEAI membership opens the door to networking opportunities and information sharing
- BEAI members have access to CPD approved events

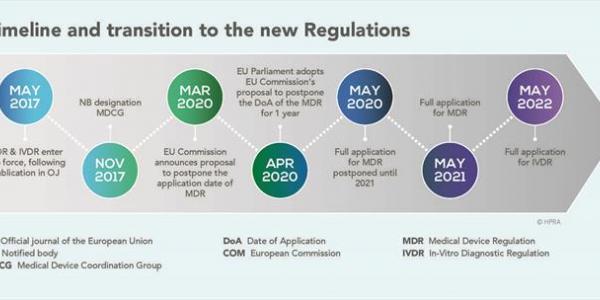
Regulation (EU) 2017/746, known as the In Vitro Diagnostic Medical Devices Regulation (IVDR), comes into effect in the European Union, 26 May 2022.
The IVDR changes and strengthens the regulatory system for in vitro diagnostic medical devices (IVDs) in the EU. IVDs are devices used to perform tests on biological samples to determine the status of a person’s health. IVDs include a broad range of devices, from self-tests for pregnancy and blood glucose tests for diabetics, to sophisticated diagnoses performed in clinical laboratories.
The IVDR will ensure that IVDs meet high standards of safety and effectiveness for the people who use them. It will do this by creating a more effective, transparent and robust system for regulating IVDs across Europe.
The IVDR will complement the Medical Devices Regulation (MDR), which came into effect in 2021 to improve the regulation of medical devices. Together, the IVDR and the MDR replace the rules created in the 1990s and respond to the substantial technological and scientific progress in the devices sector in recent years.
As the new legislation is in the form of a Regulation rather than a Directive, it can be applied directly at national level. This allows for greater legal certainty and prevents variation in the rules for IVDs across EU Member States. This means that the same high standards of safety and effectiveness will apply to all IVDs available in the EU.
Key changes to ensure safety and effectiveness of IVDs can be found by visiting the HPRA website here
GCEA and IFMBE Clinical Engineering Division are conducting a survey that aims to identify cloud connectivity preferences for surgical robotics systems, especially with regard to privacy and cybersecurity.
By answering this survey, you earn a chance to win one of three USD $100 Gift Cards, so don't miss out!
Visit https://site.globalcea.org/surgical-robotics-connectivity-survey to complete
When you think of global health, who comes to your mind? I bet you thought of doctors, nurses, public health specialists, disease detectives and academic researchers. You probably did not think of engineers. That is because they generally stay out of the limelight, but silently keep things powered and running in the background - from water and sanitation systems in the community, to ventilators and biomedical devices in hospitals.
To read the full article in Forbes magazine visit here
AI is a field of science, which combines computer science and robust datasets, to enable problem-solving. It makes it possible for machines to learn from experience, adjust to new inputs and perform human-like tasks. AI is being rapidly deployed in many areas of the healthcare landscape. AI solutions have the potential to help improve outcomes in healthcare by guiding informed decisions at critical steps of the patient journey. This event will primarily focus on the Healthcare Providers, attracting healthcare c- suite executives and Informatics Heads from leading hospitals who will assess the business value outcomes of AI and share experiences of implementation in hospital operations. Leading AI companies and eminent speakers from different countries are welcome to join this Series. As an attendee, one gets to understand the broader picture on How AI in Transforming the Healthcare and How AI is playing a Key role in the Modern Healthcare and Hospital Management, AI in Disease Diagnosis, AI In Medical Imaging, How AI is used in Surgery and Patient Safety and many other topics.
To participate in the AI in Healthcare & Hospital Management 2022 web series hosted by SK Global Events visit here
ISO/IEC/JTC 1/WG 12 - 3D Printing and Scanning, are seeking experts to participate on the drafting of a standard which provides an overview of the segmentation process for medical image-based modelling of human bone. The draft is attached for your consideration.
If any experts on medical imaging are interested in participating please contact Mr Aditya Mohan at aditya.mohan@nsai.ie for further information.
Please feel free to pass this call for experts on to any colleagues you believe may be interested in participating.

For further information, or if you would like to get in touch with the BEAI, please contact comms@beai.ie
News
About Us
The Biomedical / Clinical Engineering Association of Ireland (BEAI) is a company limited by guarantee and not having a share capital. Company Registration no. 484921.
Stay Connected on:
Important Information
Contact Us
For general information, including registration, please contact us at:
- 8 Priory Office Park, Stillorgan Road,
- Blackrock, Co Dublin, Ireland,
- A94 EE95
